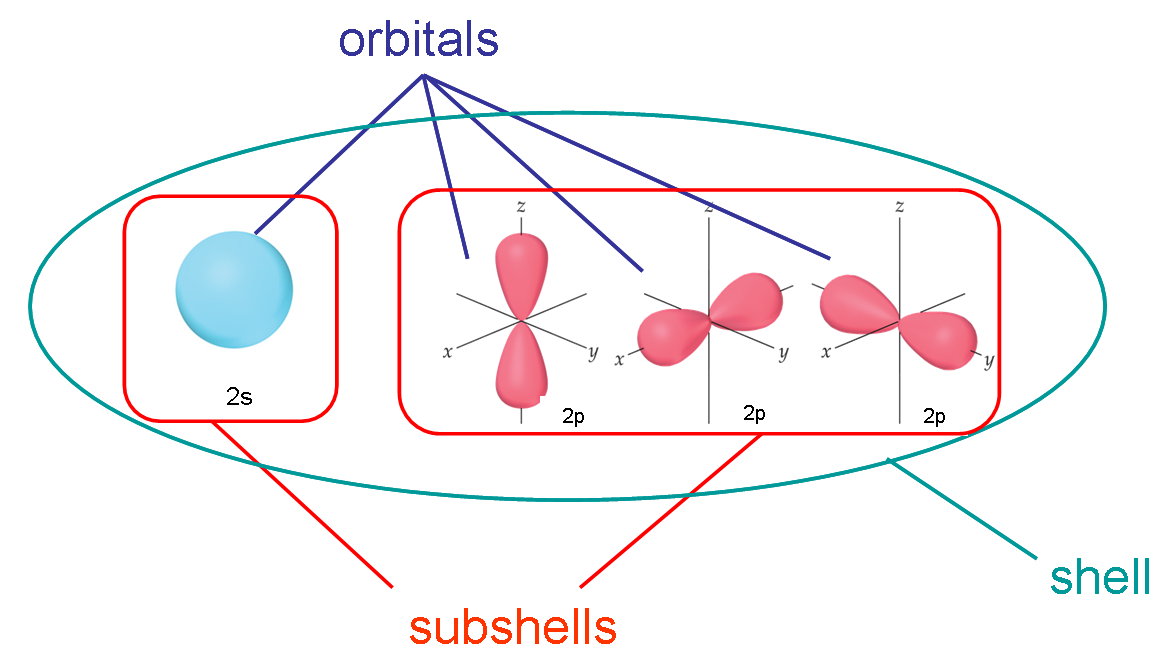
What Does Electron Shell Refer To . if we imagine an atom with one additional electron, we end up with an atom that has a single electron in its outer. an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Shells have stationary energy levels, the energy of each shell is constant. what is an electron shell with a model and diagram. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. Each stationary orbit or shell is associated with a definite amount of energy. an electron shell, also known as a main energy level, is a group of atomic orbitals with the same value of the principal. Learn how many electrons are found in each shell with the.
from socratic.org
Shells have stationary energy levels, the energy of each shell is constant. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. what is an electron shell with a model and diagram. an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. an electron shell, also known as a main energy level, is a group of atomic orbitals with the same value of the principal. if we imagine an atom with one additional electron, we end up with an atom that has a single electron in its outer. Learn how many electrons are found in each shell with the. According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. Each stationary orbit or shell is associated with a definite amount of energy.
What is the difference between electron shells and electron orbitals
What Does Electron Shell Refer To According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. an electron shell, also known as a main energy level, is a group of atomic orbitals with the same value of the principal. Each stationary orbit or shell is associated with a definite amount of energy. According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. what is an electron shell with a model and diagram. if we imagine an atom with one additional electron, we end up with an atom that has a single electron in its outer. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Learn how many electrons are found in each shell with the. an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Shells have stationary energy levels, the energy of each shell is constant.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table What Does Electron Shell Refer To what is an electron shell with a model and diagram. Each stationary orbit or shell is associated with a definite amount of energy. an electron shell, also known as a main energy level, is a group of atomic orbitals with the same value of the principal. an electron shell is a collection of orbitals that share the. What Does Electron Shell Refer To.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Does Electron Shell Refer To Learn how many electrons are found in each shell with the. Shells have stationary energy levels, the energy of each shell is constant. an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known. What Does Electron Shell Refer To.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5413898 What Does Electron Shell Refer To According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Learn how many electrons are found in each shell with the. Each stationary orbit or shell is. What Does Electron Shell Refer To.
From www.scienceabc.com
Octet Rule Definition, Explanation, Exceptions And Examples What Does Electron Shell Refer To if we imagine an atom with one additional electron, we end up with an atom that has a single electron in its outer. Each stationary orbit or shell is associated with a definite amount of energy. what is an electron shell with a model and diagram. an electron shell, also known as a main energy level, is. What Does Electron Shell Refer To.
From www.pinterest.com.au
Nitrogen(N) electron configuration with full orbital diagram Nitrogen What Does Electron Shell Refer To if we imagine an atom with one additional electron, we end up with an atom that has a single electron in its outer. According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons.. What Does Electron Shell Refer To.
From www.scienceabc.com
What Are Valence Electrons and How To Find Them? Where Are They Located? What Does Electron Shell Refer To Each stationary orbit or shell is associated with a definite amount of energy. what is an electron shell with a model and diagram. According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. an electron shell is a collection of orbitals that share the same principal. What Does Electron Shell Refer To.
From facts.net
8 Fascinating Facts About Electron Shell What Does Electron Shell Refer To Learn how many electrons are found in each shell with the. an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Each stationary orbit or shell is associated with a definite amount of energy. Shells have stationary energy levels, the energy of each shell is constant. electron shell,. What Does Electron Shell Refer To.
From edu.rsc.org
How to teach electron configurations Poster RSC Education What Does Electron Shell Refer To Learn how many electrons are found in each shell with the. what is an electron shell with a model and diagram. According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. an electron shell is a collection of orbitals that share the same principal quantum number,. What Does Electron Shell Refer To.
From socratic.org
What is the difference between electron shells and electron orbitals What Does Electron Shell Refer To Shells have stationary energy levels, the energy of each shell is constant. if we imagine an atom with one additional electron, we end up with an atom that has a single electron in its outer. Learn how many electrons are found in each shell with the. an electron shell is a collection of orbitals that share the same. What Does Electron Shell Refer To.
From www.worksheetsplanet.com
What Is An Electron What Does Electron Shell Refer To Shells have stationary energy levels, the energy of each shell is constant. Learn how many electrons are found in each shell with the. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Each stationary orbit or shell is associated with a definite amount of energy. if we imagine an atom with one additional electron,. What Does Electron Shell Refer To.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram What Does Electron Shell Refer To Shells have stationary energy levels, the energy of each shell is constant. what is an electron shell with a model and diagram. if we imagine an atom with one additional electron, we end up with an atom that has a single electron in its outer. an electron shell is a collection of orbitals that share the same. What Does Electron Shell Refer To.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table What Does Electron Shell Refer To Shells have stationary energy levels, the energy of each shell is constant. what is an electron shell with a model and diagram. Each stationary orbit or shell is associated with a definite amount of energy. an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. electron shell,. What Does Electron Shell Refer To.
From www.nagwa.com
Lesson Video Electron Shells Nagwa What Does Electron Shell Refer To an electron shell, also known as a main energy level, is a group of atomic orbitals with the same value of the principal. Each stationary orbit or shell is associated with a definite amount of energy. what is an electron shell with a model and diagram. if we imagine an atom with one additional electron, we end. What Does Electron Shell Refer To.
From www.nagwa.com
Question Video Determining What Forms When a Sodium Atom Loses One What Does Electron Shell Refer To According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. Learn how many electrons are found in each shell with the. an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. an electron shell, also known. What Does Electron Shell Refer To.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) What Does Electron Shell Refer To an electron shell, also known as a main energy level, is a group of atomic orbitals with the same value of the principal. Shells have stationary energy levels, the energy of each shell is constant. what is an electron shell with a model and diagram. According to bohr’s atomic model electrons revolve around the nucleus in a specific. What Does Electron Shell Refer To.
From mmerevise.co.uk
Atoms Questions and Revision MME What Does Electron Shell Refer To if we imagine an atom with one additional electron, we end up with an atom that has a single electron in its outer. Shells have stationary energy levels, the energy of each shell is constant. an electron shell, also known as a main energy level, is a group of atomic orbitals with the same value of the principal.. What Does Electron Shell Refer To.
From proper-cooking.info
Electron Shell Diagram What Does Electron Shell Refer To Learn how many electrons are found in each shell with the. According to bohr’s atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Each stationary orbit or shell is associated with a definite amount of energy.. What Does Electron Shell Refer To.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Does Electron Shell Refer To an electron shell, also known as a main energy level, is a group of atomic orbitals with the same value of the principal. what is an electron shell with a model and diagram. an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Shells have stationary energy. What Does Electron Shell Refer To.